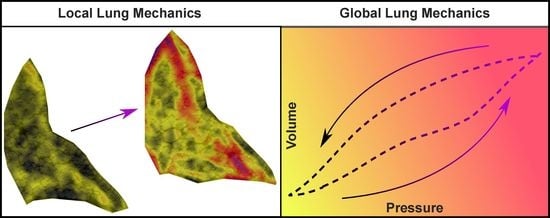
Correlating Local Volumetric Tissue Strains with Global Lung Mechanics Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lung Sample Preparation
2.3. Lung Mechanics Measurements
2.3.1. Controlled inflation Tester (CiT)
2.3.2. Protocol for Pressure-Volume (P-V) Measurements during SR-micro-CT Imaging
2.4. Image Acquisition
2.5. Image Analysis: Reconstruction and Filtering
2.6. Image Analysis: Digital Volume Correlation (DVC)
3. Results
3.1. Imaging Optimisation
3.2. Zero-Strain Test and the Reliability of the DVC Results
3.3. Local vs. Global Mechanics Highlighted by DVC and P-V
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tawhai, M.H.; Lin, C.-L. Image-based modeling of lung structure and function. J. Magn. Reson. Imaging 2010, 32, 1421–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; Cetto, R.; Doorly, D.; Bates, A.J.; Rose, J.N.; McIntyre, C.; Comerford, A.; Madani, G.; Tolley, N.S.; Schroter, R. Assessing Changes in Airflow and Energy Loss in a Progressive Tracheal Compression Before and After Surgical Correction. Ann. Biomed. Eng. 2020, 48, 822–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, S.; Sakamoto, T.; Sera, T.; Yokota, H.; Ono, K.; Doorly, D.J.; Schroter, R.C.; Tanaka, G. Voxel-based modeling of airflow in the human nasal cavity. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, A.J.; Comerford, A.; Cetto, R.; Doorly, D.J.; Schroter, R.C.; Tolley, N.S. Computational fluid dynamics benchmark dataset of airflow in tracheas. Data Brief 2017, 10, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, H.; Takaki, R.; Suki, B. A three-dimensional model of the human airway tree. J. Appl. Physiol. 1999, 87, 2207–2217. [Google Scholar] [CrossRef]
- Leary, D.; Winkler, T.; Braune, A.; Maksym, G.N. Effects of airway tree asymmetry on the emergence and spatial persistence of ventilation defects. J. Appl. Physiol. 2014, 117, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.K.; Mansy, H.A.; Gamage, P.T. Geometric features of pig airways using computed tomography. Physiol. Rep. 2016, 4, e12995. [Google Scholar] [CrossRef]
- Eskandari, M.; Pfaller, M.R.; Kuhl, E. On the Role of Mechanics in Chronic Lung Disease. Materials 2013, 6, 5639–5658. [Google Scholar] [CrossRef] [Green Version]
- Eskandari, M.; Nordgren, T.M.; O’Connell, G.D. Mechanics of pulmonary airways: Linking structure to function through constitutive modeling, biochemistry, and histology. Acta Biomater. 2019, 97, 513–523. [Google Scholar] [CrossRef]
- Sera, T.; Yokota, H.; Uesugi, K.; Yagi, N. Airway distension during lung inflation in healthy and allergic-sensitised mice in vivo. Respir. Physiol. Neurobiol. 2013, 185, 639–646. [Google Scholar] [CrossRef]
- Tawhai, M.H.; Burrowes, K.S.; Hoffman, E.A. Computational models of structure-function relationships in the pulmonary circulation and their validation. Exp. Physiol. 2006, 91, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Litzlbauer, H.D.; Korbel, K.; Kline, T.L.; Jorgensen, S.M.; Eaker, D.R.; Bohle, R.M.; Ritman, E.L.; Langheinrich, A.C. Synchrotron-Based Micro-CT Imaging of the Human Lung Acinus. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2010, 293, 1607–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sera, T.; Yokota, H.; Tanaka, G.; Uesugi, K.; Yagi, N.; Schroter, R.C. Murine pulmonary acinar mechanics during quasi-static inflation using synchrotron refraction-enhanced computed tomography. J. Appl. Physiol. 2013, 115, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, H.; Nila, A.; Vitharana, K.; Sherwood, J.M.; Nguyen, T.-T.N.; Karunaratne, A.; Mohammed, I.K.; Bodey, A.J.; Hellyer, P.J.; Overby, D.R.; et al. Microstructural Consequences of Blast Lung Injury Characterized with Digital Volume Correlation. Front. Mater. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Ranunkel, O.; Güder, F.; Arora, H. Soft Robotic Surrogate Lung. ACS Appl. Biol. Mater. 2019, 2, 1490–1497. [Google Scholar] [CrossRef] [Green Version]
- Denny, E.; Schroter, R.C. Viscoelastic Behavior of a Lung Alveolar Duct Model. J. Biomech. Eng. 1999, 122, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mishima, M.; Hirai, T.; Itoh, H.; Nakano, Y.; Sakai, H.; Muro, S.; Nishimura, K.; Oku, Y.; Chin, K.; Ohi, M.; et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 1999, 96, 8829–8834. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, D.E.; Villarroel, N.; Retamal, J.; Bugedo, G.; Bruhn, A. Improving the Accuracy of Registration-Based Biomechanical Analysis: A Finite Element Approach to Lung Regional Strain Quantification. IEEE Trans. Med Imaging 2016, 35, 580–588. [Google Scholar] [CrossRef]
- Broche, L.; Pisa, P.; Porra, L.; Degrugilliers, L.; Bravin, A.; Pellegrini, M.; Borges, J.B.; Perchiazzi, G.; Larsson, A.; Hedenstierna, G.; et al. Individual Airway Closure Characterized In Vivo by Phase-Contrast CT Imaging in Injured Rabbit Lung*. Crit. Care Med. 2019, 47, e774–e781. [Google Scholar] [CrossRef]
- Hurtado, D.E.; Erranz, B.; Lillo, F.; Sarabia-Vallejos, M.; Iturrieta, P.; Morales, F.; Blaha, K.; Medina, T.; Diaz, F.; Cruces, P. Progression of regional lung strain and heterogeneity in lung injury: Assessing the evolution under spontaneous breathing and mechanical ventilation. Ann. Intensiv. Care 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Thurgood, J.; Dubsky, S.; Uesugi, K.; Curtis, M.; Samarage, C.R.; Thompson, B.R.; Zosky, G.R.; Fouras, A. Imaging lung tissue oscillations using high-speed X-ray velocimetry. J. Synchrotron Radiat. 2016, 23, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Sera, T.; Uesugi, K.; Yagi, N.; Yokota, H. Numerical simulation of airflow and microparticle deposition in a synchrotron micro-CT-based pulmonary acinus model. Comput. Methods Biomech. Biomed. Eng. 2014, 18, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, K.; Nishimoto, K.; Ii, S.; Sera, T.; Wada, S. Heterogeneous structure and surface tension effects on mechanical response in pulmonary acinus: A finite element analysis. Clin. Biomech. 2019, 66, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ara, E.; Peña-Fernández, M.; Palanca, M.; Giorgi, M.; Cristofolini, L.; Tozzi, G. (3 more authors) Precision of Digital Volume Correlation Approaches for Strain Analysis in Bone Imaged with Micro-Computed Tomography at Different Dimensional Levels. Front. Mater. 2017, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Coudrillier, B.; Geraldes, D.M.; Vo, N.T.; Atwood, R.; Reinhard, C.; Campbell, I.C.; Raji, Y.; Albon, J.; Abel, R.L.; Ethier, C.R. Phase-Contrast Micro-Computed Tomography Measurements of the Intraocular Pressure-Induced Deformation of the Porcine Lamina Cribrosa. IEEE Trans. Med Imaging 2016, 35, 988–999. [Google Scholar] [CrossRef]
- Palanca, M.; Cristofolini, L.; Dall’Ara, E.; Curto, M.; Innocente, F.; Danesi, V.; Tozzi, G. Digital volume correlation can be used to estimate local strains in natural and augmented vertebrae: An organ-level study. J. Biomech. 2016, 49, 3882–3890. [Google Scholar] [CrossRef] [Green Version]
- Danesi, V.; Tozzi, G.; Cristofolini, L. Application of digital volume correlation to study the efficacy of prophylactic vertebral augmentation. Clin. Biomech. 2016, 39, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Tavana, S.; Clark, J.; Prior, J.; Baxan, N.; Masouros, S.; Newell, N.; Hansen, U. Quantifying deformations and strains in human intervertebral discs using Digital Volume Correlation combined with MRI (DVC-MRI). J. Biomech. 2020, 102, 109604. [Google Scholar] [CrossRef]
- Fernández, M.P.; Barber, A.; Blunn, G.; Tozzi, G. Optimization of digital volume correlation computation in SR-microCT images of trabecular bone and bone-biomaterial systems. J. Microsc. 2018, 272, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.P.; Cipiccia, S.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Pani, M.; Blunn, G.W.; Barber, A.H.; Tozzi, G. Effect of SR-microCT radiation on the mechanical integrity of trabecular bone using in situ mechanical testing and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2018, 88, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Madi, K.; Staines, K.A.; Bay, B.K.; Javaheri, B.; Geng, H.; Bodey, A.J.; Cartmell, S.; Pitsillides, A.A.; Lee, P.D. In situ characterization of nanoscale strains in loaded whole joints via synchrotron X-ray tomography. Nat. Biomed. Eng. 2020, 4, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.V.A.; Flechas, G.M.; Molimard, J.; Avril, S. Three-Dimensional Full-Field Strain Measurements across a Whole Porcine Aorta Subjected to Tensile Loading Using Optical Coherence Tomography–Digital Volume Correlation. Front. Mech. Eng. 2018, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Levchuk, A.; Schneider, P.; Meier, M.; Vogel, P.; Donaldson, F.; Müller, R. An Automated Step-Wise Micro-Compression Device for 3D Dynamic Image-Guided Failure Assessment of Bone Tissue on a Microstructural Level Using Time-Lapsed Tomography. Front. Mater. 2018, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Bay, B.K.; Smith, T.S.; Fyhrie, D.P.; Saad, M. Digital volume correlation: Three-dimensional strain mapping using X-ray tomography. Exp. Mech. 1999, 39, 217–226. [Google Scholar] [CrossRef]
- Mitzner, W.; Brown, R.; Lee, W. In vivo measurement of lung volumes in mice. Physiol. Genom. 2001, 4, 215–221. [Google Scholar] [CrossRef]
- Barré, S.F.; Haberthür, D.; Stampanoni, M.; Schittny, J.C. Efficient estimation of the total number of acini in adult rat lung. Physiol. Rep. 2014, 2, e12063. [Google Scholar] [CrossRef] [Green Version]
- Pešić, Z.; De Fanis, A.; Wagner, U.; Rau, C. Experimental stations at I13 beamline at Diamond Light Source. J. Phys. 2013, 425, 182003. [Google Scholar] [CrossRef]
- Rau, C.; Wagner, U.; Pešić, Z.; De Fanis, A. Coherent imaging at the Diamond beamline I13. Phys. Status Solidi 2011, 208, 2522–2525. [Google Scholar] [CrossRef]
- Atwood, R.C.; Bodey, A.J.; Price, S.W.T.; Basham, M.; Drakopoulos, M. A high-throughput system for high-quality tomographic reconstruction of large datasets at Diamond Light Source. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140398. [Google Scholar] [CrossRef]
- Wadeson, N.; Basham, M. Savu: A Python-based, MPI Framework for Simultaneous Processing of Multiple, N-dimensional. Large Tomography Datasets. arXiv 2016, arXiv:1610.08015. [Google Scholar]
- Vo, N.T.; Atwood, R.C.; Drakopoulos, M. Radial lens distortion correction with sub-pixel accuracy for X-ray micro-tomography. Opt. Express 2015, 23, 32859–32868. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.T.; Atwood, R.C.; Drakopoulos, M. Superior techniques for eliminating ring artifacts in X-ray micro-tomography. Opt. Express 2018, 26, 28396–28412. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, G.; Fernández, M.P.; Davis, S.; Karali, A.; Kao, A.P.; Blunn, G.W. Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation. Materials 2020, 13, 2579. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.P.; Kao, A.P.; Witte, F.; Arora, H.; Tozzi, G. Low-cycle full-field residual strains in cortical bone and their influence on tissue fracture evaluated via in situ stepwise and continuous X-ray computed tomography. J. Biomech. 2020, 113, 110105. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Hooper, P.; Dear, J.P. The Effects of Air and Underwater Blast on Composite Sandwich Panels and Tubular Laminate Structures. Exp. Mech. 2011, 52, 59–81. [Google Scholar] [CrossRef] [Green Version]
- Arora, H.; Hooper, P.; Dear, J. Dynamic response of full-scale sandwich composite structures subject to air-blast loading. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1651–1662. [Google Scholar] [CrossRef] [Green Version]
- Arora, H.; Kelly, M.; Worley, A.; Fergusson, A.; Dear, J.P. Compressive strength after blast of sandwich composite materials. Phil. Trans. R. Soc. A 2014, 372, 20130212. [Google Scholar] [CrossRef] [Green Version]
- Arora, H.; Hooper, P.; Del Linz, P.; Yang, H.; Chen, S.; Dear, J.P. Modelling the behaviour of composite sandwich structures when subject to air-blast loading. Int. J. Multiphysics 2012, 6, 199–218. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.; Arora, H.; Worley, A.; Kaye, M.; Del Linz, P.; Hooper, P.A.; Dear, J.P. Sandwich panel cores for blast applications: Materials and graded density. Exp. Mech. 2015, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Arora, H.; Del Linz, P.; Dear, J.P. Damage and deformation in composite sandwich panels exposed to multiple and single explosive blasts. Int. J. Impact Eng. 2017, 104, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, E.; Quinn, R.; Sancho, A.; Johnson, A.; Liu, H.; Hooper, P.A.; Dear, J.P.; Arora, H. Blast resilience of composite sandwich panels with hybrid glass-fibre and carbon-fibre skins. Multiscale Multidiscip. Modeling Exp. Des. 2018, 1, 197–210. [Google Scholar]
- Rolfe, E.; Quinn, R.; Irven, G.; Brick, D.; Dear, J.P.; Arora, H. Underwater blast loading of partially submerged sandwich composite materials in relation to air blast loading response. Int. J. Light. Mater. Manuf. 2020, 3, 387–402. [Google Scholar] [CrossRef]
- Mariano, C.A.; Sattari, S.; Maghsoudi-Ganjeh, M.; Tartibi, M.; Lo, D.D.; Eskandari, M. Novel Mechanical Strain Characterization of Ventilated ex vivo Porcine and Murine Lung using Digital Image Correlation. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, H.; Mitchell, R.L.; Johnston, R.; Manolesos, M.; Howells, D.; Sherwood, J.M.; Bodey, A.J.; Wanelik, K. Correlating Local Volumetric Tissue Strains with Global Lung Mechanics Measurements. Materials 2021, 14, 439. https://doi.org/10.3390/ma14020439
Arora H, Mitchell RL, Johnston R, Manolesos M, Howells D, Sherwood JM, Bodey AJ, Wanelik K. Correlating Local Volumetric Tissue Strains with Global Lung Mechanics Measurements. Materials. 2021; 14(2):439. https://doi.org/10.3390/ma14020439
Chicago/Turabian StyleArora, Hari, Ria L. Mitchell, Richard Johnston, Marinos Manolesos, David Howells, Joseph M. Sherwood, Andrew J. Bodey, and Kaz Wanelik. 2021. "Correlating Local Volumetric Tissue Strains with Global Lung Mechanics Measurements" Materials 14, no. 2: 439. https://doi.org/10.3390/ma14020439