Enhanced Electrical Conductivity and Seebeck Coefficient in PEDOT:PSS via a Two-Step Ionic liquid and NaBH4 Treatment for Organic Thermoelectrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
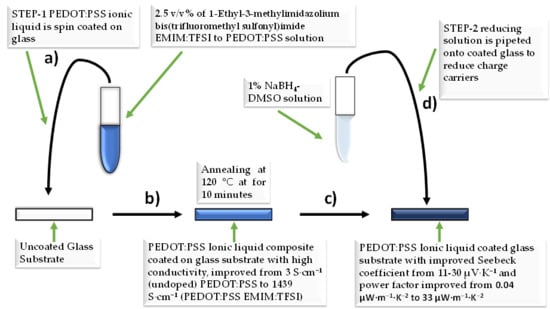
2.2. Fabrication of PEDOT:PSS EMIM:TFSI Composite Films
2.3. Fabrication of PEDOT:PSS EMIM:TFSI-NaBH4 Composite Films
2.4. Characterization
3. Results and Discussion
3.1. Thermoelectric Performance of PEDOT:PSS EMIM:TFSI Composite Films
3.2. Post Treatment of EMIM:TFSI Films with NaBH4
3.3. Mechanisms for the Improved Electrical Conductivity of EMIM:TFSI and EMIM:TFSI NaBH4 Films
3.4. Mechanisms for the Improved Seebeck Coefficient of EMIM:TFSI NaBH4 Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bubnova, O.; Crispin, X. Towards polymer-based organic thermoelectric generators. Energy Environ. Sci. 2012, 5, 9345. [Google Scholar] [CrossRef] [Green Version]
- Cowen, L.M.; Atoyo, J.; Carnie, M.J.; Baran, D.; Schroeder, B.C. Review—Organic Materials for Thermoelectric Energy Generation. ECS J. Solid State Sci. Technol. 2017, 6, N3080–N3088. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yu, C. Organic Thermoelectrics: Materials Preparation, Performance Optimization, and Device Integration. Joule 2019, 3, 53–80. [Google Scholar] [CrossRef] [Green Version]
- Culebras, M.; Choi, K.; Cho, C. Recent Progress in Flexible Organic Thermoelectrics. Micromachines 2018, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Recent progress on PEDOT-based thermoelectric materials. Materials 2015, 8, 732–750. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Katz, H.E.; Fang, F.; Opila, R.L. Promising thermoelectric properties of commercial PEDOT:PSS materials and their Bi2 Te3 powder composites. ACS Appl. Mater. Interfaces 2010, 2, 3170–3178. [Google Scholar] [CrossRef]
- Smith, P.M.; Su, L.; Gong, W.; Nakamura, N.; Reeja-Jayan, B.; Shen, S. Thermal conductivity of poly(3,4-ethylenedioxythiophene) films engineered by oxidative chemical vapor deposition (oCVD). RSC Adv. 2018, 8, 19348–19352. [Google Scholar] [CrossRef] [Green Version]
- Kee, S.; Kim, H.; Paleti, S.H.K.; El Labban, A.; Neophytou, M.; Emwas, A.-H.; Alshareef, H.N.; Baran, D. Highly Stretchable and Air-Stable PEDOT:PSS/Ionic Liquid Composites for Efficient Organic Thermoelectrics. Chem. Mater. 2019. [Google Scholar] [CrossRef]
- Huang, J.; Miller, P.F.; Wilson, J.S.; de Mello, A.J.; de Mello, J.C.; Bradley, D.D.C. Investigation of the effects of doping and post-deposition treatments on the conductivity, morphology, and work function of poly(3,4-ethylenedioxythiophene)/poly(styrene sulfonate) films. Adv. Funct. Mater. 2005, 15, 290–296. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Tsai, T.C.; Chang, H.C.; Chen, C.H.; Whang, W.T. Widely variable Seebeck coefficient and enhanced thermoelectric power of PEDOT:PSS films by blending thermal decomposable ammonium formate. Org. Electron. Phys. Mater. Appl. 2011, 12, 2159–2164. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.G.; Hong, J.I.; Kim, S.H.; Kwak, J. Sulfuric acid vapor treatment for enhancing the thermoelectric properties of PEDOT:PSS thin-films. J. Mater. Sci. Mater. Electron. 2016, 27, 6122–6127. [Google Scholar] [CrossRef]
- Saxena, N.; Keilhofer, J.; Maurya, A.K.; Fortunato, G.; Overbeck, J. Facile Optimization of Thermoelectric Properties in PEDOT:PSS Thin Films through Acido-Base and Redox Dedoping Using Readily Available Salts. ACS Appl. Energy Mater. 2018. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.H.; Kim, F.S.; Choi, H.H.; Cheong, I.W.; Kim, J.H. Enhanced thermoelectric properties of PEDOT:PSS nanofilms by a chemical dedoping process. J. Mater. Chem. A 2014, 2, 6532–6539. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Chen, C.H.; Boopathi, K.M.; Pranoto, F.W.; Li, L.J.; Chu, C.W. Enhanced thermoelectric performance of PEDOT:PSS flexible bulky papers by treatment with secondary dopants. ACS Appl. Mater. Interfaces 2015, 7, 94–100. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]
- Luo, J.; Billep, D.; Waechtler, T.; Otto, T.; Toader, M.; Gordan, O.; Sheremet, E.; Martin, J.; Hietschold, M.; Zahn, D.R.T.; et al. Enhancement of the thermoelectric properties of PEDOT:PSS thin films by post-treatment. J. Mater. Chem. A 2013, 1, 7576–7583. [Google Scholar] [CrossRef]
- Döbbelin, M.; Marcilla, R.; Salsamendi, M.; Pozo-Gonzalo, C.; Carrasco, P.M.; Pomposo, J.A.; Mecerreyes, D. Influence of Ionic Liquids on the Electrical Conductivity and Morphology of PEDOT:PSS Films. Chem. Mater. 2007, 19, 2147–2149. [Google Scholar] [CrossRef]
- Kee, S.; Kim, N.; Kim, B.S.; Park, S.; Jang, Y.H.; Lee, S.H.; Kim, J.; Kim, J.; Kwon, S.; Lee, K. Controlling Molecular Ordering in Aqueous Conducting Polymers Using Ionic Liquids. Adv. Mater. 2016, 28, 8625–8631. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Jiang, Q.; Shi, H.; Jiang, F.; Xu, J.; Xiong, J.; Liu, E. Optimizing the thermoelectric properties of PEDOT:PSS films by combining organic co-solvents with inorganic base. J. Mater. Sci. Mater. Electron. 2015, 26, 8515–8521. [Google Scholar] [CrossRef]
- Massonnet, N.; Carella, A.; Jaudouin, O.; Rannou, P.; Laval, G.; Celle, C.; Simonato, J.-P. Improvement of the Seebeck coefficient of PEDOT:PSS by chemical reduction combined with a novel method for its transfer using free-standing thin films. J. Mater. Chem. C 2014, 2, 1278. [Google Scholar] [CrossRef]
- Badre, C.; Marquant, L.; Alsayed, A.M.; Hough, L.A. Highly Conductive Poly (3,4-ethylenedioxythiophene ):Poly(styrenesulfonate) Films Using 1-Ethyl-3-methylimidazolium Tetracyanoborate Ionic Liquid. Adv. Funct. Mater. 2012, 22, 2723–2727. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2017, 3, 1–15. [Google Scholar] [CrossRef]
- Chou, T.R.; Chen, S.H.; Chiang, Y.T.; Lin, Y.T.; Chao, C.Y. Highly Conductive PEDOT:PSS Film by Post-Treatment with Dimethyl Sulfoxide for ITO-Free Liquid Crystal Display. Mol. Cryst. Liq. Cryst. 2015, 612, 201–210. [Google Scholar] [CrossRef]
- Wang, J.; Cai, K.; Shen, S. A facile chemical reduction approach for effectively tuning thermoelectric properties of PEDOT films. Org. Electron. 2015, 17, 151–158. [Google Scholar] [CrossRef]
- Wang, X.; Meng, F.; Tang, H.; Gao, Z.; Li, S.; Jiang, F.; Xu, J. An effective dual-solvent treatment for improving the thermoelectric property of PEDOT:PSS with white graphene. J. Mater. Sci. 2017, 52, 9806–9818. [Google Scholar] [CrossRef]
- Beretta, D.; Barker, A.J.; Maqueira-Albo, I.; Calloni, A.; Bussetti, G.; Dell’Erba, G.; Luzio, A.; Duò, L.; Petrozza, A.; Lanzani, G.; et al. Thermoelectric Properties of Highly Conductive Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate Printed Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 18151–18160. [Google Scholar] [CrossRef]
- Fan, Z.; Du, D.; Yao, H.; Ouyang, J. Higher PEDOT Molecular Weight Giving Rise to Higher Thermoelectric Property of PEDOT:PSS: A Comparative Study of Clevios P and Clevios PH1000. ACS Appl. Mater. Interfaces 2017, 9, 11732–11738. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Wang, J.; Zhang, J.; Yang, Q. Solar Energy Materials & Solar Cells Highly conductive PEDOT: PSS transparent electrode prepared by a post-spin-rinsing method for ef fi cient ITO-free polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 143–149. [Google Scholar]
- Photovoltaics, I.O. Unraveling the Enhanced Electrical Conductivity of PEDOT:PSS Thin Films for Unraveling the Enhanced Electrical Conductivity of PEDOT:PSS Thin Films for ITO-Free Organic Photovoltaics. IEEE Photonics J. 2014. [Google Scholar] [CrossRef]
- Lenz, A.; Kariis, H.; Pohl, A.; Persson, P.; Ojamäe, L. The electronic structure and reflectivity of PEDOT: PSS from density functional theory. Chem. Phys. 2011, 384, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.S.; Oh, T.H.; Kim, S.H.; Han, S.S.; Lee, S.J.; Lee, S.G.; Lee, Y.J.; Jang, S.S. Effect of solvent on electrical conductivity and gas sensitivity of PEDOT: PSS polymer composite films. J. Appl. Polym. Sci. 2015, 132, 2–7. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Feng, G.; Ge, M. On the mechanism of conductivity enhancement in PEDOT:PSS/PVA blend fiber induced by UV-light irradiation. Appl. Phys. A 2020, 126, 184. [Google Scholar] [CrossRef]
- Saghaei, J.; Fallahzadeh, A.; Yousefi, M.H. Highly Conductive Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Films Using 1-Ethyl-3-methylimidazolium Tetracyanoborate Ionic Liquid. Org. Electron. Phys. Mater. Appl. 2015, 19, 70–75. [Google Scholar]
- Teo, M.Y.; Kim, N.; Kee, S.; Kim, B.S.; Kim, G.; Hong, S.; Jung, S.; Lee, K. Highly stretchable and highly conductive PEDOT:PSS/Ionic liquid composite transparent electrodes for solution-processed stretchable electronics. ACS Appl. Mater. Interfaces 2017, 9, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, J.; Chen, S.; Zou, J.; Xie, W.; Zeng, X. Highly Conductive PEDOT:PSS Transparent Hole Transporting Layer with Solvent Treatment for High Performance Silicon/Organic Hybrid Solar Cells. Nanoscale Res. Lett. 2017, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Jang, J.G.; Kim, J.-G.; Hong, J.-I.; Kim, J.; Kwak, J.; Kim, S.H.; Shin, S. Structural and Morphological Evolution for Water-resistant Organic Thermoelectrics. Sci. Rep. 2017, 7, 13287. [Google Scholar] [CrossRef] [Green Version]
- Greczynski, G.; Kugler, T.; Salaneck, W.R. Characterization of the PEDOT-PSS system by means of X-ray and ultraviolet photoelectron spectroscopy. Thin Solid Films 1999, 354, 129–135. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.; Chu, C. Highly conductive PEDOT:PSS treated with formic acid for ITO-free polymer solar cells Highly Conductive PEDOT:PSS Treated with Formic Acid for ITO-Free Polymer Solar Cells. ACS Appl. Mater. Interfaces 2014. [Google Scholar] [CrossRef]
- Khan, M.A.; Armes, S.P.; Perruchot, C.; Ouamara, H.; Chehimi, M.M.; Greaves, S.J.; Watts, J.F. Surface Characterization of Poly(3,4-ethylenedioxythiophene)-Coated Latexes by X-ray Photoelectron Spectroscopy. Langmuir 2000, 4171–4179. [Google Scholar] [CrossRef]
- Keppler, A.; Himmerlich, M.; Ikari, T.; Marschewski, M.; Pachomow, E.; Höfft, O.; Maus-Friedrichs, W.; Endres, F.; Krischok, S. Changes of the near-surface chemical composition of the 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide room temperature ionic liquid under the influence of irradiation. Phys. Chem. Chem. Phys. 2011, 13, 1174–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Mukaida, M.; Naitoh, Y.; Ishida, T. Morphological Change and Mobility Enhancement in PEDOT:PSS by Adding Co-solvents. Adv. Mater. 2013, 25, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- De Kok, M.M.; Buechel, M.; Vulto, S.I.E.; van de Weijer, P.; Meulenkamp, E.A.; de Winter, S.H.P.M.; Mank, A.J.G.; Vorstenbosch, H.J.M.; Weijtens, C.H.L.; van Elsbergen, V. Modification of PEDOT:PSS as hole injection layer in polymer LEDs. Phys. Status Solidi 2004, 1359, 1342–1359. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atoyo, J.; Burton, M.R.; McGettrick, J.; Carnie, M.J. Enhanced Electrical Conductivity and Seebeck Coefficient in PEDOT:PSS via a Two-Step Ionic liquid and NaBH4 Treatment for Organic Thermoelectrics. Polymers 2020, 12, 559. https://doi.org/10.3390/polym12030559
Atoyo J, Burton MR, McGettrick J, Carnie MJ. Enhanced Electrical Conductivity and Seebeck Coefficient in PEDOT:PSS via a Two-Step Ionic liquid and NaBH4 Treatment for Organic Thermoelectrics. Polymers. 2020; 12(3):559. https://doi.org/10.3390/polym12030559
Chicago/Turabian StyleAtoyo, Jonathan, Matthew R. Burton, James McGettrick, and Matthew J. Carnie. 2020. "Enhanced Electrical Conductivity and Seebeck Coefficient in PEDOT:PSS via a Two-Step Ionic liquid and NaBH4 Treatment for Organic Thermoelectrics" Polymers 12, no. 3: 559. https://doi.org/10.3390/polym12030559