Bonding of Gold Nanoclusters on Graphene with and without Point Defects
Abstract
:1. Introduction
2. Materials and Methods
3. Results
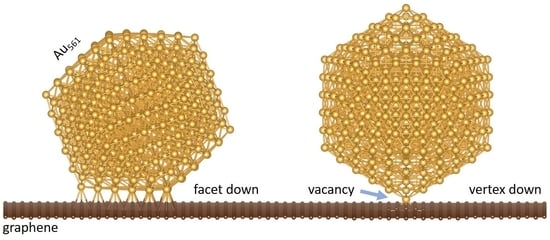
3.1. Nanoclusters with a Facet on Graphene
3.2. Nanoclusters with a Vertex on Graphene
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Mahmood, N.; Zhu, J.; Hou., Y.; Sun., S. Graphene and its Composites with Nanoparticles for Electrochemical Energy Applications. Nano Today 2014, 9, 668–683. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.B. Design, Synthesis, and Characterization of Graphene-nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.C.; Crerana, B.; Rotelloa, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.T. Using Gold Nanoparticles for Catalysis. Nano Today 2007, 2, 40–43. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Edwards, J.K. Chapter 6—Application of gold nanoparticles in catalysis. In Frontiers of Nanoscience; Elsevier Ltd.: Oxford, UK, 2012; Volume 3, pp. 249–293. [Google Scholar] [CrossRef]
- Muszynski, R.; Seger, B.; Kamat, P.V. Decorating Graphene Sheets with Gold Nanoparticles. J. Phys. Chem. C 2008, 14, 5263–5266. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, H.; Wu, Z.; Li, X.; He, Y.; Yuan, Z. The Comparison of Different Gold Nanoparticles Graphene Nanosheets Hybrid Nanocomposites in Electrochemical Performance and the Construction of a Sensitive Uric Acid Electrochemical Sensor with Novel Hybrid Nanocomposites. Biosens. Bioelectron 2011, 29, 102–108. [Google Scholar] [CrossRef]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag-Au Nanoparticles Coated Graphene Electrochemical Sensor for Ultrasensitive Analysis of Carcinoembryonic Antigen in Clinical Immunoassay. Sens. Actuators B-Chem. 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Torres-Mendieta, R.; Ventura-Espinosa, D.; Sabater, S.; Lancis, J.; Mínguez-Vega, G.; Mata, J.A. In Situ Decoration of Graphene Sheets with Gold Nanoparticles Synthetized by Pulsed Laser Ablation in Liquids. Sci. Rep. 2016, 6, 30478. [Google Scholar] [CrossRef] [Green Version]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.V.; Karpan, M.; van den Brink, J.; Kelly, P.J. Doping Graphene with Metal Contacts. Phys. Rev. Lett. 2008, 101, 026803. [Google Scholar] [CrossRef]
- Sundaram, R.S.; Steiner, M.; Chiu, H.Y.; Engel, M.; Bol, A.A.; Krupke, R.; Burghard, M.; Kern, K.; Avouris, P. The Graphene-gold Interface and its Implications for Nanoelectronics. Nano Lett. 2011, 11, 3833–3837. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Gimenez-Lopez, M.d.C.; Fay, M.W.; Herreros Lucas, C.; Brown, P.D.; Khlobystov, A.N. Dynamics of Gold Nanoparticles on Carbon Nanostructures Driven by Van Der Waals and Electrostatic Interactions. Small 2015, 11, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Claeyssens, F.; Pratontep, S.; Xirouchaki, C.; Palmer, R.E. Immobilization of Large Size-selected Silver Clusters on Graphite. Nanotechnology 2006, 17, 805. [Google Scholar] [CrossRef]
- Rodríguez-Zamora, P.; Yin, F.; Palmer, R.E. Enhanced Immobilization of Gold Nanoclusters on Graphite. J. Phys. Chem. A 2014, 118, 8182–8187. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa-Abad, J.A.; Soldano, G.J.; Mejía-Rosales, S.J.; Mariscal, M.M. Immobilization of Au Nanoparticles on Graphite Tunnels Through Nanocapillarity. RSC Adv. 2016, 81, 77195–77200. [Google Scholar] [CrossRef] [Green Version]
- Pulido, A.; Boronat, M.; Corma, A. Theoretical Investigation of Gold Clusters Supported on Graphene Sheets. New J. Chem. 2011, 35, 2153–2161. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-initio Total Energy Calculations for Metals and Semiconductors Using a Plane-wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab initio Total-energy Calculations Using a Plane-wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 1396. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector Augmented-wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Wells, D.M.; Rossi, G.; Ferrando, R.; Palmer, R.E. Metastability of the Atomic Structures of Size-selected Gold Nanoparticles. Nanoscale 2015, 15, 6498–6503. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, C.R.C.; Oliveira, L.N.; Tereshchuk, P.; Da Silva, J.L.F. Comparative Study of Van Der Waals Corrections to the Bulk Properties of Graphite. J. Phys. Cond. Matter 2015, 27, 415502. [Google Scholar]
- Buimaga-Iarinca, L.; Morari, C. Adsorption of Small Aromatic Molecules on Gold: A DFT Localized Basis Set Study Including Van Der Waals Effects. Theor. Chem. Acc. 2014, 133, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Reyes, J.C.F.; Siler, C.G.F.; Liu, W.; Tkatchenko, A.; Friend, C.M.; Madix, R.J. Van Der Waals Interactions Determine Selectivity in Catalysis by Metallic Gold. J. Am. Chem. Soc. 2014, 136, 13333–13340. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.M.; Balbás, L.C. GGA Versus Van Der Waals Density Functional Results for Mixed Gold/mercury Molecules and Pure Au and Hg Cluster Properties. Phys. Chem. Chem. Phys. 2011, 13, 20863–20870. [Google Scholar] [CrossRef]
- Reimers, J.R.; Ford, M.J.; Marcuccio, S.M.; Ulstrup, J.; Hush, N.S. Competition of Van Der Waals and Chemical Forces on Gold–sulfur Surfaces and Nanoparticles. Nat. Rev. Chem. 2017, 1, 0017. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for Three-dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zenodo. Available online: https://doi.org/10.5281/zenodo.4116312 (accessed on 21 October 2020).
- Skowron, S.T.; Lebedeva, I.V.; Popov, A.M.; Bichoutskaia, E. Energetics of Atomic Scale Structure Changes in Graphene. Chem. Soc. Rev. 2015, 44, 3143–3176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pašti, I.A.; Jovanović, A.; Dobrota, A.S.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Atomic Adsorption on Graphene with a Single Vacancy: Systematic DFT Study Through the Periodic Table of Elements. Phys. Chem. Chem. Phys. 2018, 20, 858–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, L.; Pedersen, A.; Gao, Y.; Khetrapal, N.; Jónsson, J.; Zeng, X.C. Magic-number Gold Nanoclusters with Diameters From 1 to 3.5 nm: Relative Stability and Catalytic Activity for CO Oxidation. Nano Lett. 2015, 15, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.M.; Ferrando, R.; Palmer, R.E. Experimental Determination of the Energy Difference Between Competing Isomers of Deposited Size-selected Gold Nanoclusters. Nat. Commun. 2018, 9, 1323. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Molnár, G.; Pálinkás, A.; Deák, A.; Osváth, Z. Vapour Sensing Properties of Graphene-covered Gold Nanoparticles. Nanoscale Adv. 2019, 1, 2408–2415. [Google Scholar] [CrossRef] [Green Version]
- Farokhnezhad, M.; Esmaeilzadeh, M. Graphene Coated Gold Nanoparticles: An Emerging Class of Nanoagents for Photothermal Therapy Applications. Phys. Chem. Chem. Phys. 2019, 21, 18352–18362. [Google Scholar] [CrossRef]
- Osváth, Z.; Deák, A.; Kertész, K.; Molnár, G.; Vértesy, G.; Zámbó, D.; Hwang, C.; Biróac, L.P. The Structure and Properties of Graphene on Gold Nanoparticle. Nanoscale 2015, 7, 5503–5509. [Google Scholar] [CrossRef] [Green Version]








| NP Shape | Pristine Graphene | Graphene with Vacancy |
|---|---|---|
| Cuboctahedral | −5.81 | −5.98 |
| Decahedral | −6.52 | −6.74 |
| Icosahedral | −6.90 | −7.14 |
| NP Shape | Pristine Graphene | Graphene with Vacancy |
|---|---|---|
| Cuboctahedral | −2.14 | −5.21 |
| Decahedral | −2.53 | −5.51 |
| Icosahedral | −2.51 | −5.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavloudis, T.; Kioseoglou, J.; Palmer, R.E. Bonding of Gold Nanoclusters on Graphene with and without Point Defects. Nanomaterials 2020, 10, 2109. https://doi.org/10.3390/nano10112109
Pavloudis T, Kioseoglou J, Palmer RE. Bonding of Gold Nanoclusters on Graphene with and without Point Defects. Nanomaterials. 2020; 10(11):2109. https://doi.org/10.3390/nano10112109
Chicago/Turabian StylePavloudis, Theodoros, Joseph Kioseoglou, and Richard E. Palmer. 2020. "Bonding of Gold Nanoclusters on Graphene with and without Point Defects" Nanomaterials 10, no. 11: 2109. https://doi.org/10.3390/nano10112109