Abstract
Highly productive tropical seagrasses often live adjacent to or among coral reefs and utilize large amounts of inorganic carbon. In this study, the effect of seagrass productivity on seawater carbonate chemistry and coral calcification was modelled on the basis of an analysis of published data.
Published data (11 studies, 64 records) reveal that seagrass meadows in the Indo-Pacific have an 83% chance of being net autotrophic, resulting in an average net sink of 155 gC m−2 yr−1. The capacities for seagrass productivity were analysed using an empirical model to examine the effect on seawater carbonate chemistry. Our analyses indicate that increases in pH of up to 0.38 units, and Ωarag increases of 2.9 are possible in the presence of seagrass meadows (compared to their absence) with the precise values of these increases dependent on water residence time (tidal flushing) and water depth. In shallow water reef environments, Scleractinian coral calcification downstream of seagrass has the potential to be ≈18% greater than in an environment without seagrass. If this potential benefit to reef calcifiers is supported by further study it offers a potential tool in marine park management at a local scale. The applicability of this will depend upon local physical conditions as well as the spatial configuration of habitats, and the factors that influence their productivity. This novel study suggests that, in addition to their importance to fisheries, sediment stabilization and primary production, seagrass meadows may enhance coral reef resilience to future ocean acidification.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Managing coral reef ecosystems for future environmental change is focused on improving ecosystem resilience to high-intensity, short-term stressors such as elevated temperature [1, 2]. Appropriate and effective management responses to the more gradual process of ocean acidification (OA) will require actions that potentially break from tradition and may require extensive ecosystem intervention [3]. Seagrasses and other primary producers (namely macro-algae), through their capacity to cycle (and export from a reef system) large amounts of dissolved inorganic carbon [4, 5], may have a key role to play in localized management responses to the impacts of OA [6, 7].
OA results from the addition of anthropogenic carbon to seawater, which shifts the dissolved inorganic carbon (DIC) equilibrium to a state of reduced carbonate saturation [8]. This reduces calcification and growth rates of Scleractinian corals [9, 10], potentially leading to significant long-term consequences for coral-dependent species [11]. Global models of future OA are largely based on oceanic carbonate dynamics and do not consider the high variability of carbonate chemistry within shallow water systems and the role that benthic marine organisms, particularly primary producers, have upon driving such variability in these systems [7, 12]. Photosynthetic uptake of DIC by primary producers results in a displacement of the DIC equilibrium in the opposite direction to that imposed by OA and leads to increased  concentration [13]. Research has also revealed that while coral dominated habitats are a net source of CO2, potentially increasing the impacts of OA, macro-algae dominated reef-tops potentially increase seawater pH by reducing CO2 through photosynthetic uptake [7]. Similar to macro-algae, seagrasses take up DIC (as both CO2 and
concentration [13]. Research has also revealed that while coral dominated habitats are a net source of CO2, potentially increasing the impacts of OA, macro-algae dominated reef-tops potentially increase seawater pH by reducing CO2 through photosynthetic uptake [7]. Similar to macro-algae, seagrasses take up DIC (as both CO2 and  ) and can displace the carbonate equilibrium. Studies in the Philippines [14], the Great Barrier Reef [15] and the Mediterranean [16], have recorded diel changes in pH of between 0.5 and 0.7 units in seagrass habitats, with peak values (leading to higher carbonate saturation) being reached around mid-day when photosynthesis is at a maximum. These changes may affect
) and can displace the carbonate equilibrium. Studies in the Philippines [14], the Great Barrier Reef [15] and the Mediterranean [16], have recorded diel changes in pH of between 0.5 and 0.7 units in seagrass habitats, with peak values (leading to higher carbonate saturation) being reached around mid-day when photosynthesis is at a maximum. These changes may affect  uptake and calcification rates of other organisms (see figure 1). This is a process demonstrated at the micro-scale within small mesocosms but not yet at a habitat scale [17–19].
uptake and calcification rates of other organisms (see figure 1). This is a process demonstrated at the micro-scale within small mesocosms but not yet at a habitat scale [17–19].
Figure 1. The key biological processes interacting to alter seagrass communities from being net autotrophic to net heterotrophic.
Download figure:
Standard imageAs one of the most productive marine habitats [5], the capacity of seagrass meadows to alter carbonate chemistry is particularly pertinent given that they form habitat mosaics with coral reefs throughout the tropics [20]. Within many shallow fringing reef-flat environments, corals are commonly found in close association with seagrasses, either as isolated colonies, or forming a gradient of habitat from reef to seagrass [21, 22]. Seagrasses also provide a range of valuable ecosystem services, and have the potential to increase their productivity in a high CO2 world [23]. Understanding the interaction of seagrass meadows with seawater carbonate chemistry is also important given their role in carbon sequestration and the implications of this for future blue carbon frameworks [4, 5].
For seagrass to offset OA and increase the calcification rates of proximal corals, requires that seagrass meadows are in a state of net autotrophy. That is, photosynthetic DIC utilization exceeds respiration rates, not just in the seagrass plants, but in their benthic and epiphytic faunal communities. During active photosynthetic production, DIC is consumed and pH increases, resulting in a diel cycle in pH [13, 14, 24]. Other conditions affecting meadow-scale productivity status include: seasonal changes in seagrass productivity and biomass [25]; changes in faunal and periphyton abundance [26]; carbonate dissolution rates that alter total alkalinity (TA) in underlying sediments [27]; and the amount of seagrass biomass degrading within the meadow [28] (figure 1). Temporal and spatial variability are therefore necessary considerations in the assessment of seagrass–seawater interactions. Whether such productivity will influence seawater chemistry (both within the seagrass meadow and that of adjacent habitat) will be dependent upon the complex nature of the hydrodynamic regime (e.g. depth, residence time, and its mixing), the capacity for the air–sea interface to modify these changes [12] and the spatial arrangement of habitats relative to the hydrodynamic regime.
We conduct an analysis of seagrass meadows in the Indo-Pacific to provide a theoretical demonstration of the premise that seagrasses in this region are sufficiently net autotrophic to alter seawater carbonate chemistry at a meadow scale and are therefore capable of locally offsetting the effects of OA.
2. Methods
The present study utilized all known literature data sources of seagrass community metabolism in the tropical Indo-Pacific to determine their productivity status. This was conducted to provide a theoretical demonstration of the influence of this productivity on seawater carbonate chemistry. The dataset (see supplementary information available at stacks.iop.org/ERL/7/024026/mmedia) is taken from 11 separate studies comprising 64 individual records conducted across Australia, Indonesia, Thailand, Sri Lanka, India, Philippines, Fiji and Kenya. All records are taken from original peer reviewed studies with the exception of 2 records of unpublished data contained within the dataset collated by [5]. All data are changes in oxygen concentration within the water column, representing net oxygen release. Of the 64 records, 41 are from studies that utilized 'in situ benthic chambers', while 17 were determined according to the 'in situ diel curve procedure'. Six records are from studies that utilized benthic chambers but conducted the measurements within the laboratory. While such a dataset provides limitations due to varied methodologies and lack of temporal and spatial resolution, we consider it to be of sufficient value in providing a first observational dataset, although the conclusions from this review will require further field validation.
Gas measurements have been criticized for underestimating productivity, as oxygen produced during photosynthetic C uptake can be transferred into the rhizome and sediments. Also, factors such as photorespiration, leakage of dissolved organic carbon, and the formation or translocation of carbon reserves may cause a discrepancy between net photosynthesis and actual growth [29]. However, the method gives a reasonable estimate of productivity with a possible underestimation of 10–15% [29, 30]. This suggests that our analysis is therefore a conservative estimate of productivity, especially given recent studies indicating that water flow increases seagrass photosynthesis, and that many of these studies had restricted water flow within measurement chambers [31]. Not all original data sources were in mgC, so oxygen values were converted to carbon equivalents using a conversion factor of 0.31 [32].
2.1. Analysis of seagrass production over annual and seasonal timescales
Net community productivity in seagrass meadows is defined here as the difference between uptake of carbon (by photosynthetic organisms and physical processes in the seagrass meadow), and carbon release (by all organisms and physical processes in the community) during respiration [5]. Its quantification, on an annual scale, requires consideration of diurnal and seasonal variability. To date, such studies that document temporal variability are largely limited to temperate or sub-tropical regions [12, 25]. Although some data on Indo-Pacific tropical seagrass productivity exist, these are mostly temporally discrete studies based on low sample numbers, which lack sufficient temporal resolution to estimate annual meadow trophic status.
To provide an estimate of annual net productivity, we sought to understand seasonal variability in productivity. All available regional data sources for community productivity (including all flora and fauna within a seagrass meadow) (see supplementary information available at stacks.iop.org/ERL/7/024026/mmedia) were plotted relative to time of the year and split into seasons (austral summer and winter: October to March, and April to September). This enabled daily mean values to be determined for the austral winter and austral summer, and an estimated value for annual community productivity (mgC m−2 yr−1) to be calculated. Statistical differences were tested with one-way ANOVA on Ranks in SigmaPlot V11 following failure of normality tests.
2.2. Modelling of seawater carbonate chemistry
A simple empirical model (table 1) was developed to determine how each individual value of net productivity within each season might influence seawater DIC on a daily basis at a range of possible dilutions (based on depth and water residence time) to represent typical seagrass meadows in the Indo-Pacific (see supplementary information available at stacks.iop.org/ERL/7/024026/mmedia). The influence of carbon addition (or loss) to both a 1 and 5 m column (i.e. 1 and 5 m3) of water was modelled (based on assumed complete mixing of the water column). These depths represent typical depth ranges of productive fringing reef seagrass in the Indo-Pacific. The intention of this simple modelling approach is to provide indicative evidence for future validation of how seagrass productivity might drive changes in shallow water DIC.
Table 1. Empirical model used to predict change in seagrass meadow seawater DIC.
| Equation | |
|---|---|
![$C=B-\left [\frac{P}{V}d\right ]$](https://content.cld.iop.org/journals/1748-9326/7/2/024026/revision1/erl431825ieqn5.gif)
|
|
| Parameters | Description |
| C | Final seagrass meadow seawater DIC (μmolC l−1) |
| B | Background seawater DIC (μmolC l−1) |
| P | Seagrass net productivity (μmol C m−2 day−1) |
| V | Volume of receiving water (l) |
| D | Daily dilution rate/residence time of receiving water (h day−1) |
The impact of lateral transport was also investigated by assuming two scenarios, with 6 and 24 h seawater residence time on the seagrass/reef flat (e.g. typical fringing reef conditions) [33], and again assuming complete mixing. The model excludes the influence of the air–sea interface in resetting the carbonate system, based on the premise that at wind speeds ranging from 2–10 m s−1 the air–sea CO2 flux is only 0.1–2.0 mmol m−2 h−1 [7, 34] and is therefore negligible compared to the impact of water transport and mixing.
The influence of changes in seawater DIC, induced by seagrass productivity, on pH and Ωarag were then determined using the carbonate chemistry calculator 'CO2sys' [35]. This calculation incorporated standard equilibrium constants used to calculate carbonate chemistry of seawater [36]. For the purposes of this model we assume that any dissolution of carbonate in the sediment does not affect water column carbonate chemistry, so water column alkalinity was kept constant at a global average value of 2308 μmol kg−1 [37]. The correctness of this assumption, and implications of its possible failure are discussed below. Salinity was assumed to be constant 35 ppt [38], and an initial total DIC of 1984 μmol kg−1 was used based on global textbook values [39].
pH and Ωarag vary as a function of seasonal temperature (see table 2). Summer calculations assumed a constant temperature of 30 °C and all winter calculations a temperature of 25 °C. These temperatures are typical for Indo-Pacific shallow water environments [38, 40], but their precise values does not significantly alter the model calculations. The predominate form of calcium carbonate used in Scleractinian coral calcification is aragonite, therefore the results of these carbonate chemistry calculations are presented in terms of pH and aragonite saturation (Ωarag).
Table 2. Background constants used in seawater chemistry model.
| Season | Salinity (ppt) | Temp (°C) | TA (μmol kg−1) | DIC (μmol kg−1) | pH | Ωarag |
|---|---|---|---|---|---|---|
| Summer | 35 | 30 | 2308 | 1984 | 8.00 | 3.76 |
| Winter | 35 | 25 | 2308 | 1984 | 8.07 | 3.64 |
3. Results
3.1. Annual patterns of seagrass meadow productivity
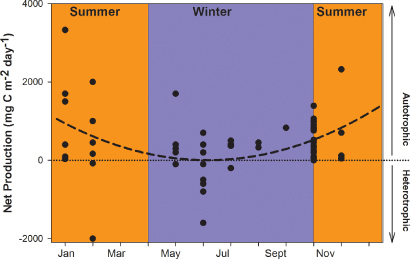
Values of seagrass community productivity from throughout the Indo-Pacific ranged from negative 2000 mgC m−2 day−1 at Groote Eylandt in Northern Australia [39], to positive 3326 mgC m−2 day−1 at Amini Atoll in India [41] with a median value of 386.5 and a mean of 509.3 ± SD1045.4 mgC m−2 day−1 (figure 2). An additional value of 5810 mgC m−2 day−1 was recorded at Kavaratti Atoll in India [42], but this was considered an outlier from the dataset and was removed from analysis. The explanation for the extreme nature of this measure from Kavaratti Atoll is not clear.
Figure 2. Net daily seagrass meadow (across species) community carbon production (mgC m−2 day−1) recorded throughout the Indo-Pacific region (data take from 11 studies, n = 64). Data is plotted relative to days in the year and fitted with a quadratic polynomial function.
Download figure:
Standard imagePlotting the data relative to time of the year demonstrates productivity to be significantly reduced (H1 = 4.7, p < 0.05) during the southern hemisphere winter in May, June and July, relative to summer in November, December and January (figure 2). The majority of the net productivity data was positive, indicating overall net autotrophic production, however during the winter 36% of measurements were negative (increasing to 54% of measurements in June and July), indicating the presence of periods of heterotrophic conditions. Overall, based on the current dataset, seagrass meadows in the Indo-Pacific have an 83% chance of being net autotrophic, with an average annual net sink of 155 ± 37 gC m−2 yr−1.
Such whole system net uptake of carbon during a complete annual cycle requires that photosynthetic DIC utilization exceeds respiration rates, not just in the seagrass plants, but in their benthic and epiphytic faunal communities, and that this carbon does not then re-enter the system via remineralization or the respiration of secondary production. Whole system net uptake requires some level of net export of this carbon from the system, either into the underlying sediment (as storage or sequestration) or out of the location of study.
This equates to a mean annual daily productivity of 0.43 ± 0.10 gC m−2 day−1. Summertime productivity was calculated as 0.60 ± 0.13 gC m−2 day−1, and wintertime productivity as 0.11 ± 0.15 gC m−2 day−1. These values for the Indo-Pacific are ≈45% higher than the global tropical average calculated at 0.24 mgC m−2 day−1 [5]. The data sources for this analysis are a more detailed review than previously available, however it supports the commonly held view that seagrass meadows are net autotrophic [5].
3.2. Influence of seagrass productivity on seawater chemistry
Net carbon uptake by seagrass meadows will lead to an increase in pH and Ωarag. Modelling these effects on seawater chemistry indicates that the pH could increase by up to 0.38 pH units (with a 24 h water residence time and 1 m depth seagrass meadow) and result in an increase in Ωarag of 2.9. These are the extreme values, with actual pH and Ωarag changing as a function of depth, water residence, temperature and seasonality.
Although large seasonal differences were observed in literature values of seagrass productivity (higher in summer), these differences do not all translate directly into pH differences in the model, principally due to the influence of temperature on pH. The positive influence of productivity on pH was greatest in the winter (figure 3). A mean (±SD) pH increase of between 0.004 ± 0.001 (5 m depth 6 h residence) and 0.072 ± 0.016 (1 m depth 24 h residence) was modelled in summer, and pH increases of between 0.005 ± 0.001 (5 m depth 6 h residence) and 0.014 ± 0.020 (1 m depth 24 h residence) modelled in winter.
Figure 3. Modelled seasonal influence of the estimated range (mean ± SD) of levels of Indo-Pacific annual seagrass net community productivity upon seawater inorganic carbon and the resultant pH changes (assuming constant total alkalinity of 2308 mmol kg−1, and salinity 35 ppm, a mean winter temperature of 25 °C, and a mean summer temperature of 30 °C). Model calculations consider seagrass meadows under two durations of water residence time (6 h and 1 day) and two water depths (assuming complete mixing of seawater). Seawater carbonate calculations use 'CO2sys' by [35], and incorporate standard equilibrium constants [36].
Download figure:
Standard imageThe value of Ωarag was lowest in winter, with mean values approximately 8% lower than summer. Summer values for Ωarag were modelled to increase by between 0.02 ± 0.01 (5 m depth 6 h residence) and 0.52 ± 0.11 (1m depth 24 h residence) while those in the winter ranged between 0.003 ± 0.006 (5 m depth 6 h residence) and 0.094 ± 0.124 (1m depth 24 h residence) (figure 3).
4. Discussion
Here we provide a first theoretical estimate, based on a collation of shorter-period studies, of the annual and seasonal habitat-scale influences of tropical seagrass productivity on seawater carbonate chemistry. Due to the largely theoretical nature of the present study and the spatial and temporal mismatch of data sources, results should be interpreted accordingly and with acknowledgment that field validation is required. However the results of our review concur with field evidence from a seasonal whole system seagrass study in the sub-tropical Mediterranean [25], and short-term diel analysis of seagrass meadow pH in the Philippines and Australia [14, 15]. The results of the present study suggest the existence of an additional functional significance of these already valuable ecosystem service providers. The findings add to data from mixed coral–algae habitats that find algal primary producers to provide similar localized OA offsetting capacity to those demonstrated here for seagrass [7].
Indo-Pacific seagrass meadows appear to be net autotrophic over an annual cycle, with their productivity altering carbonate chemistry and increasing the pH of the seawater. This subsequently has the capacity to locally (within habitat) offset the impacts of an increasingly acidic ocean by increasing the saturation state of CaCO3, which in turn is likely to enhance calcification of organisms such as coral and algae [43, 44]. We note here, that, the exact mechanism by which coral calcification is influenced by OA remains inconclusive [45]. Although the majority of evidence points to the saturation state of aragonite being the determinant of calcification rate, recent evidence suggests that this may not always be the case, and bicarbonate and pH may be more important [46].
Achieving net production (i.e. net autotrophic conditions) at annual scales requires that carbon fixed by the seagrass meadow is removed from the immediate environment in some form. This removal can happen through three routes: by direct export of organic carbon from the environment in seawater; by long-term storage and accumulation of organic carbon in the sediment underlying the seagrass meadow; or by sedimentary decay of the organic carbon and its reaction with CaCO3 in the sediment.
Depending on local hydrodynamics, seagrass derived organic carbon may be exported to other environments (e.g. the deeper ocean) to become degraded or stored [47, 48]. The extent of such export is likely to be highly site specific, but estimates of seagrass detritus loss from a reef system have been observed to be a significant proportion of productivity in some settings [28]. Recent syntheses of carbon burial within seagrass meadows have suggested that long-term sedimentary storage of organic carbon may also be a significant route for its permanent removal from the reef environment [4, 5, 49].
Some organic carbon also decays within the sediment to raise DIC and lower pH in sediment pore-waters. In the reef environment, where there is abundant carbonate in sediments, this decrease of pH can lead to an enhanced dissolution of carbonate and an increase of TA in pore-waters [27]. Due to the potential decay of organic carbon in the sediment, this may lead to a flux of DIC back to the overlying seawater. As a portion of this DIC will have been neutralized by reaction with carbonate (and resulting release of TA), this flux would still involve an increase in carbonate saturation in the water relative to simple respiration. The rate of this sedimentary release of alkalinity into the water column is poorly understood [50], but likely slow. The influence of other biological drivers of TA are poorly understood, but within system calcification by epiphytes has been shown to be an additional cause of TA flux [51, 52]. Measurement of this process cannot easily be observed due to the competing processes controlling alkalinity in the dynamic reef environment, but such neutralization of respired organic carbon by sedimentary carbonate represents a third route (along with export and burial) to support the observed net autotrophy of seagrass meadows. The release of any TA into the water column from seagrass driven sedimentary carbonate dissolution requires further study.
Our simple model estimates that seagrass productivity could, during the summer period, increase the aragonite saturation state of seawater from 3.76 to 4.27. According to laboratory-derived relationships between Ωarag and calcification rate, such an increase equates to a potential increase in calcification of between 4 and 18% [10]. Values produced by our model do not illustrate diel variability in DIC, and as a result are possibly conservative estimates upon their influence on calcification. This is because maximum productivity of a seagrass meadow loosely follows daily changes in photosynthetically active radiation (PAR). Therefore, consumption of DIC will follow PAR, resulting in highest Ωarag and potential calcification rates during the middle of the day. As calcification of corals is also light dependent [53], seagrass mediated changes in DIC may have an even greater effect on calcification than is modelled here.
Low calcification during winter [54, 55] has been attributed to reduced Ωarag rather than low temperature [56], suggesting that the summer period may be of greatest importance to the calcification of corals. The present study finds that the alteration of the Ωarag of seawater by seagrass potentially has the greatest effect in summer. Conversely if the seagrass meadows go into periods of heterotrophy during the winter period and actually produce quantities of CO2 sufficient to reduce Ωarag then the possibility exists for the increased dissolution of coral calcium carbonate [10].
Although we demonstrate seagrass mediated changes in carbonate chemistry to be a potential major annual effect, our theoretical model suggests seagrass community metabolism varies seasonally. Such variability may be in response to many local factors [25, 57] that in turn change the capacity of seagrasses to alter seawater carbonate chemistry. Surrogate measures of seasonal seagrass meadow productivity within the Indo-Pacific (i.e. seagrass growth, biomass and cover) show similar patterns of seasonality [57, 58]. This is probably a large contributor to the observed variation in net community metabolism (that includes both flora and fauna).
The present study takes a broad approach to understanding seagrass community productivity, and it is important to consider that different species assemblages will have differing effects on seawater chemistry, both directly and indirectly. Direct effects include species differences in production [59] and their sensitivity to environmental stressors [40, 60] while indirect effects include differences in the faunal assemblages that seagrass communities support [61].
Increasing water depth and mixing rates will serve to reduce the impact of net seagrass DIC uptake suggesting that seagrass meadows where water is shallow and water movement is reduced may have a higher capacity to alter seawater carbonate chemistry. In some large Indo-Pacific atoll reef systems, water residence time can be as high as 50 days [62], however smaller tidally driven residence times are most likely on small reef systems [33]. The variability in local conditions highlights the need to locally validate this concept in the field. Although there are many tropical reef systems adjacent to productive seagrass meadows where this concept has the potential to offer a novel future tool for marine park management in a high CO2 world, it is unlikely that such a premise is applicable uniformly. The applicability of this will depend upon local physical conditions, as well as the spatial configuration of habitats, and the factors that influence their productivity.
The possible increase of local TA over seagrass meadows, due to enhanced sedimentary carbonate dissolution rates [63], should also be considered in future studies, and tensioned with the impacts of other organisms (e.g. corals) which simultaneously push the carbonate equilibrium in the reverse direction by consumption of TA [7].
The ability of seagrasses to alter seawater carbonate chemistry into the future will also depend upon its capacity to remain productive in a high CO2 world, where both elevated temperature and OA are inevitable [60]. Tropical seagrasses generally have a high thermal tolerance [40, 60] but intertidal populations are influenced by high atmospheric temperatures [64]. Furthermore, a high CO2 world may actually provide conditions that will be beneficial to seagrasses [23]. Therefore, seagrass meadows are likely to remain highly productive tropical ecosystems as the climate changes, and their role in altering seawater carbonate chemistry and enhancing coral calcification rates will continue and possibly even increase.
The requirement to conserve seagrass meadows is commonly overlooked by the need to put meagre resources into charismatic habitats such as coral reefs [65, 66]. Seagrass meadows not only provide ecological functions of nursery refugia for coral reef fauna, they also provide an environment potentially beneficial for calcifying fauna, a role that is likely to increase in the future. Our review and simple modelling suggests that coral calcification in the presence of seagrass could be 18% greater than without seagrass. This study provides evidence that coral reef managers should consider seagrass conservation as a means of providing resilience to coral reef biodiversity, productivity and function.
Acknowledgments
The authors wish to thank both the Ocean Foundation and the Great Barrier Reef Foundation. RU wishes to acknowledge the support of SEACAMS at Swansea University.